Unidade de microscopia avançada e citometria de fluxo (UMACiF)
MED-UEVORA
A Unidade de Microscopia do MED oferece apoio técnico em microscopia de fluorescência e microscopia confocal, bem como na microdissecação a laser.
Equipa

Responsável
Luís Manuel Cardoso Vieira Alho

Investigador
Esther Menendez

Técnico Superior
Isabel J. P. G. Velada
Contactos
266 760 885 (ext. 44114)*
Unidade de Microscopia Avançada e Citometria de Fluxo (UMACiF)
Universidade de Évora, Pólo da Mitra, Edifício Santos Júnior, Sala 222-A, Apartado 94, 7006-554 Évora, Portugal
* Custo de chamada rede fixa nacional
EQUIPAMENTO
- Leica DM6000B Microscope: automated upright research microscope equipped with transmitted light axis and incident light axis. The microscope allows to use the main transmitted light contrast methods (bright field, dark field, phase contrast) as well as fluorescence microscopy. Objectives: 5X (air), 10X (air), 40X (air), 63X (air).
- Leica LMD6500 Laser Microdissection System: based on an upright research microscope (Leica DM6000 B, see above) it enables to isolate specific areas of soft tissues (e.g. from plant or animal tissue sections) from single cells or cell groups in a contact- and contamination- free manner. The dissected material is then directly accessible for further analyses (e.g. DNA, RNA, protein analyses). Equipped with a UV laser (355 nm). Software: Leica Laser Microdissection V 7.5.
- Leica TCS SPE Confocal System: based on a Leica DMI4000B inverted microscope, it includes 4 laser lines (405, 488, 532 and 635 nm). Objectives: 5X (air), 10X (air), 40X (oil), 63X (oil). Software: Leica LAS AF.
- Sysmex CyFlow Cube 8 Flow Cytometer: equipped with 2 lasers (blue laser 488 nm and red laser 638 nm). Optical filters: 536nm/40, 590nm/50 e 675nm/20.
- Microtome: for cutting tissue sections (2 – 60 um) of tissues (plant or animal tissue) from paraffin embedded tissue blocks
- Cryostat microtome: for cutting tissue at low temperatures (typically around −15 to −30°C).
APOIO À INVESTIGAÇÃO
UMACiF collaborates with the following research projects:
- Regacork TraDE - Transferência e Divulgação dos Estudos Técnico-Científicos da Rega de Sobreiros |ALT20-03-0246-FEDER-000061 | 01/10/2020 - 31/03/2023 | https://youtu.be/IZC7p_bqWBg?t=1089
PUBLICAÇÕES EXTERNAS NAS QUAIS OS NOSSOS EQUIPAMENTOS FORAM USADOS
- Ben Gaied, R.; Sbissi, I.; Tarhouni, M.; Brígido, C. Bacterial Endophytes from Legumes Native to Arid Environments Are Promising Tools to Improve Mesorhizobium–Chickpea Symbiosis under Salinity. Biology 2024, 13, 96. https://doi.org/10.3390/biology13020096
- Velada I, Menéndez E, Teixeira RT, Cardoso H, Peixe A. Laser Microdissection of Specific Stem-Base Tissue Types from Olive Microcuttings for Isolation of High-Quality RNA. Biology (Basel). 2021 Mar 10;10(3):209. https://doi.org/10.3390/biology10030209
- Torres DP, Paço A, Menéndez E, Mateos PF, Brígido C. Role of QseG membrane protein in beneficial enterobacterial interactions with plants and Mesorhizobia, Journal of Plant Interactions, 2021, 16:1, 510-521. https://doi.org/10.1080/17429145.2021.2000050
- Menéndez E, Pérez-Yépez J, Hernández M, Rodríguez-Pérez A, Velázquez E, León-Barrios M. Plant Growth Promotion Abilities of Phylogenetically Diverse Mesorhizobium Strains: Effect in the Root Colonization and Development of Tomato Seedlings. Microorganisms. 2020 Mar 14;8(3):412. https://doi.org/10.3390/microorganisms8030412
- da-Silva, JR, Menéndez, E, Eliziário, F., Mateos, PF, Alexandre, A, Oliveira S. Heterologous expression of nifA or nodD genes improves chickpea-Mesorhizobium symbiotic performance. Plant Soil, 2019, 436, 607–621. https://doi.org/10.1007/s11104-019-03950-0
PUBLICAÇÕES DA UMACIF
- Velada I, Alho L. 2022. Microscopia de fluorescência para deteção de fitopatogénios de espécies frutíferas mediterrânicas. Agrotec 45: 66-68.https://pt.calameo.com/read/00624353666a8a02ceb80
WORKSHOPS/APOIO À DOCÊNCIA
UMACiF collaborates with the following curricular units:
- 2023/2024: Biologia do Desenvolvimento, licenciatura em Biologia, Universidade de Évora;
- 2023/2024: Métodos e Técnicas em Biomedicina, licenciatura em Ciências Biomédicas, Universidade de Évora;
- 2021/2022: Project in Biological Sciences II (BIO12416L), degree in Biology (0602/9011), University of Évora;
- 2021/2022: Anatomy and Plant Histology (BIO12346L), degree in Biology (0602/9011), University of Évora;
- 2020/2021: Anatomy and Plant Histology (BIO12346L), degree in Biology (0602/9011), University of Évora



COMO ACEDER AOS NOSSOS EQUIPAMENTOS
Please contact Isabel Velada (ivelada@uevora.pt)
IMAGENS ADQUIRIDAS COM OS NOSSOS EQUIPAMENTOS
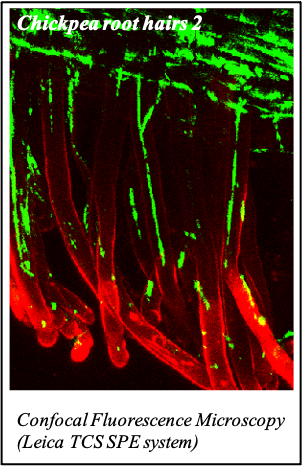
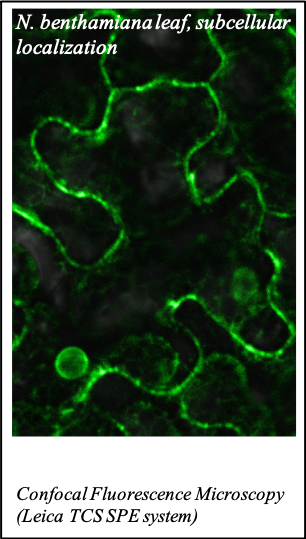
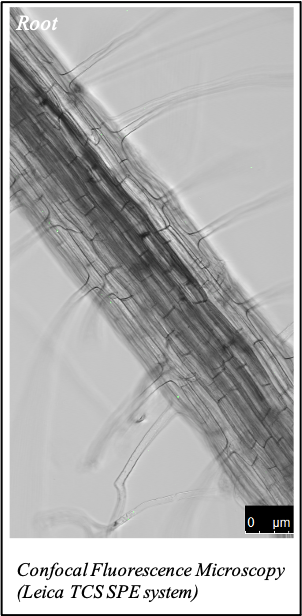
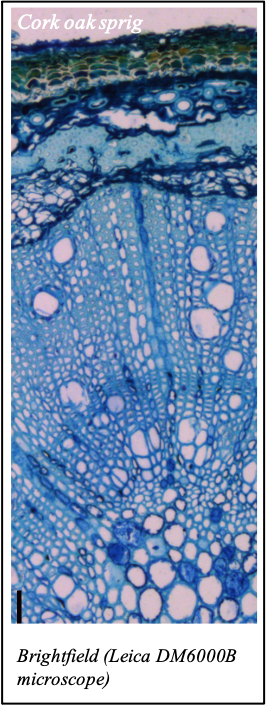
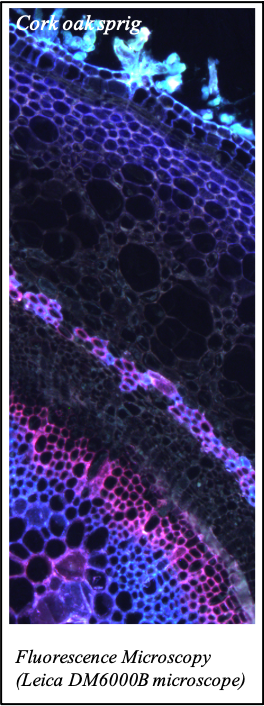
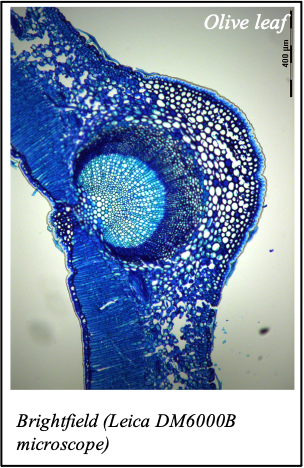
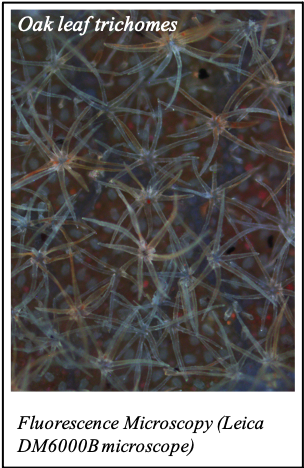
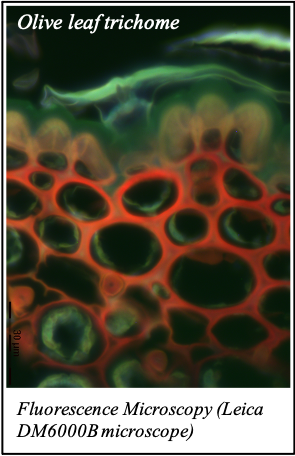
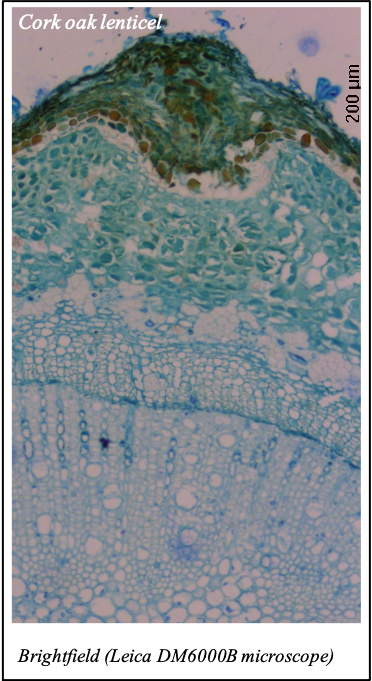
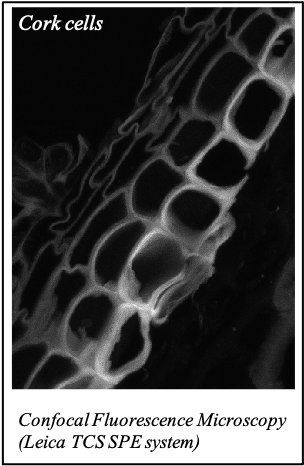
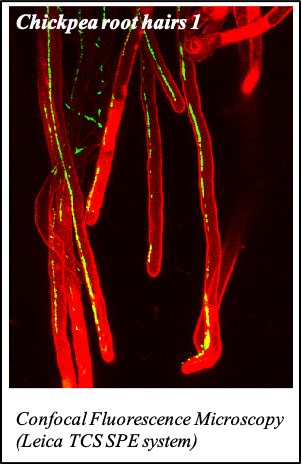
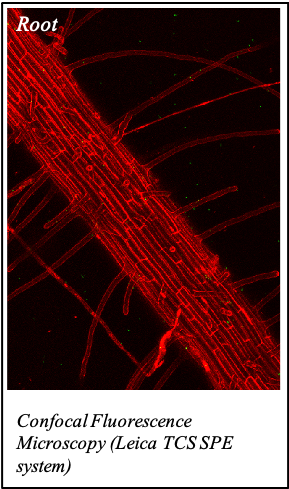
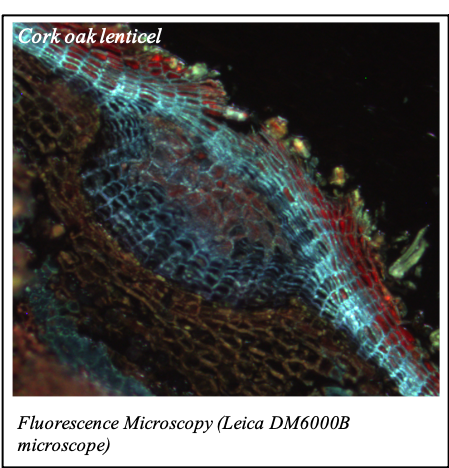
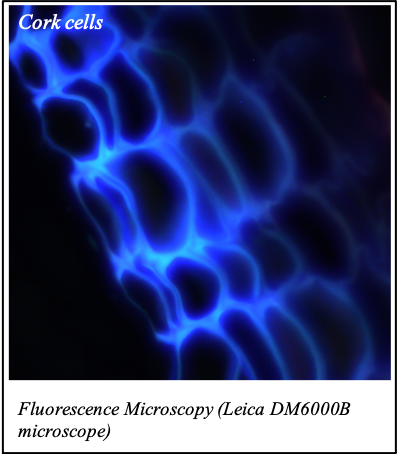
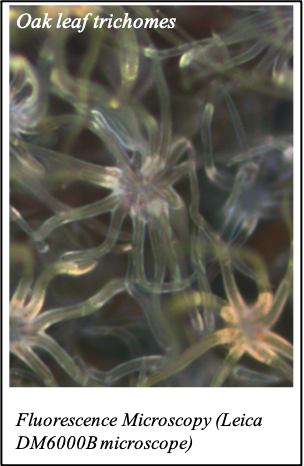
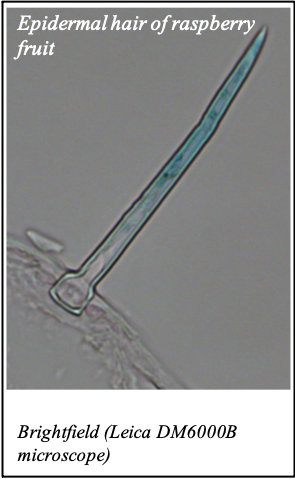
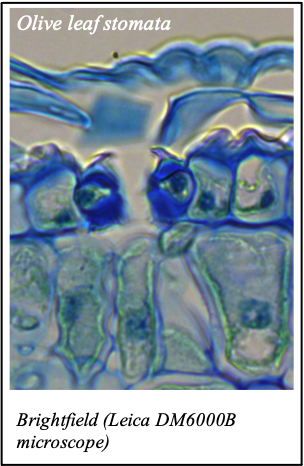
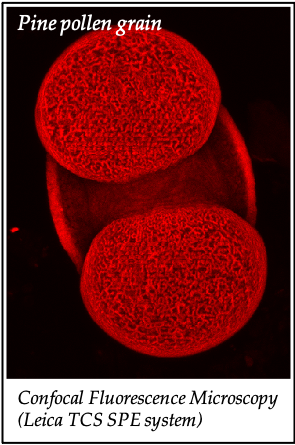
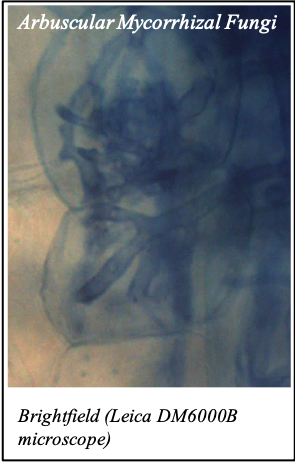
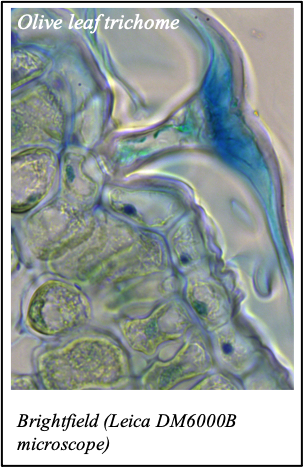
We gratefully acknowledge the following scientists for providing images and/or biological material:
'Chickpea root hairs 1' and '2', 'Root' (Dr. Clarisse Brígido and Dr. Esther Menéndez), 'N. benthamiana leaf, subcellular localization' (Dr. Cláudia Vicente Letras and Dr. Margarida Espada), 'Cork cells', 'Cork oak lenticel', Cork oak sprig' (Dr. Ana Poeiras), 'Oak leaf trichomes' (Hugo Ribeiro, MSc), 'Olive leaf' (Prof. Renato Coelho), 'Epidermal hair of raspberry fruit' (Prof. Cristina Agulheiro), 'Arbuscular Mycorrhizal Fungi' (Prof. Isabel Brito), 'Pine pollen grain' (Dr. Elsa Caeiro).